SEE
Discover and Classify Every Connected Asset in Healthcare
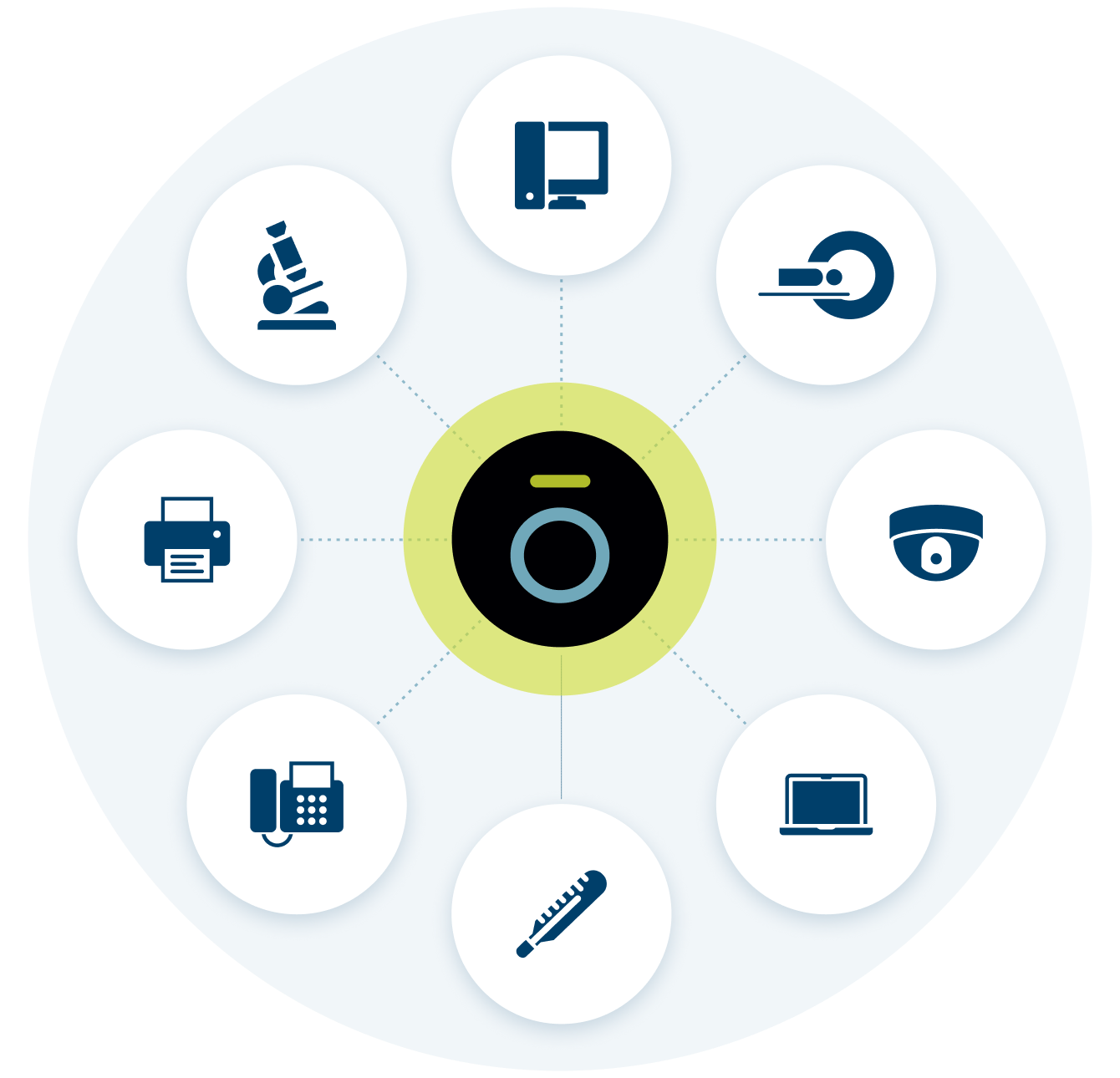
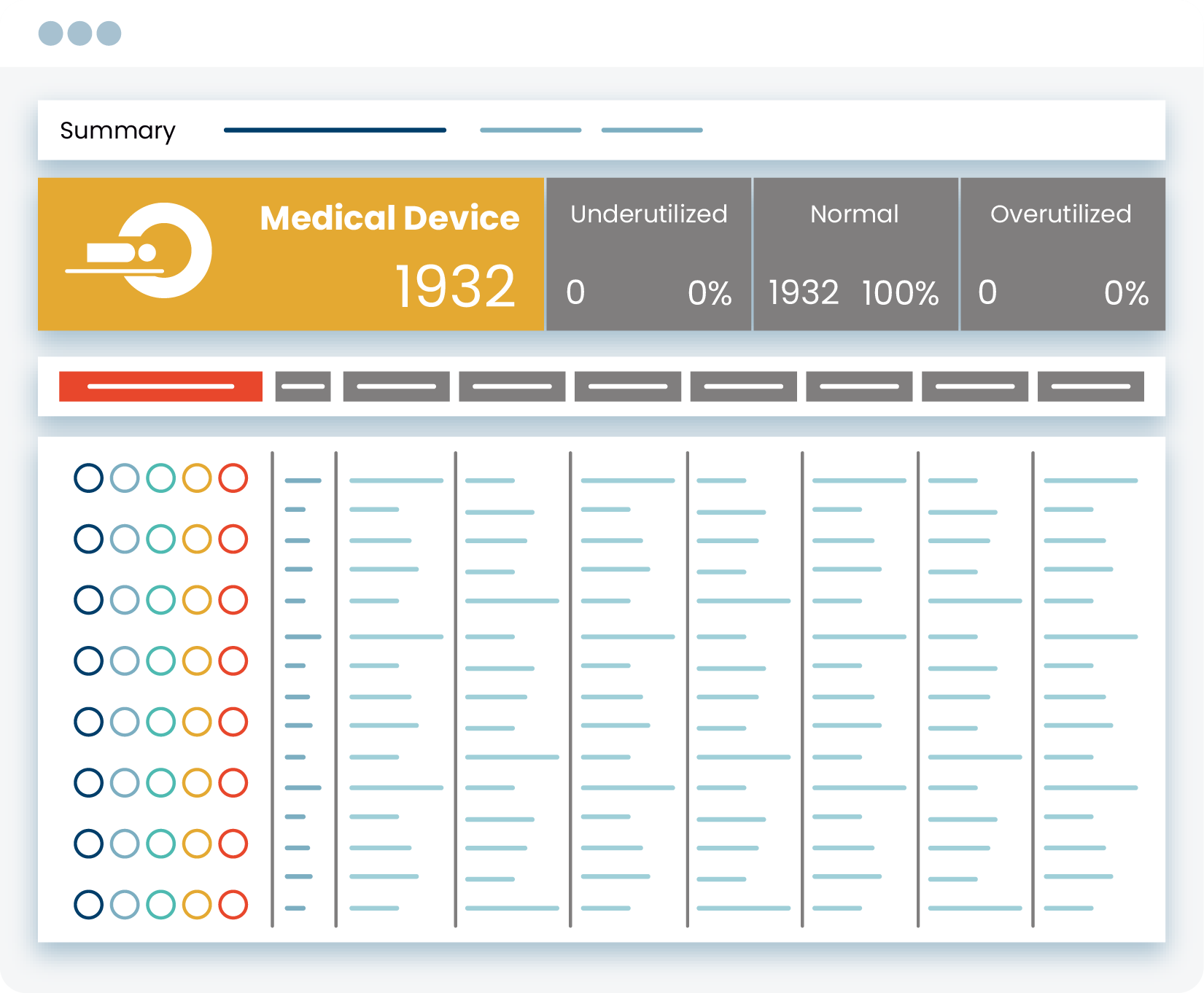
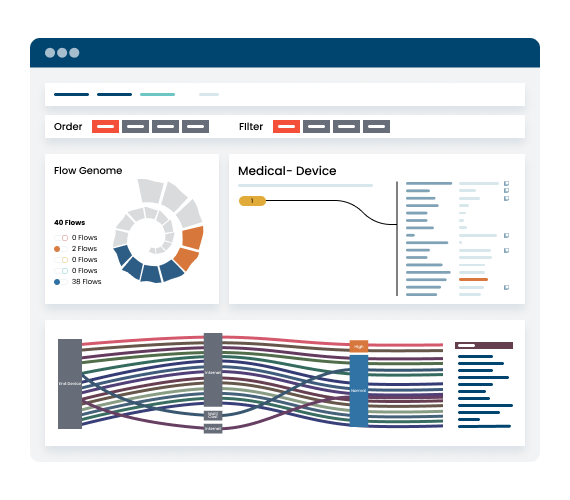
Security begins with visibility. Ordr discovers every asset including devices (IT, IOT, IOMT, OT), SaaS and cloud across all your locations, via an agentless approach. See granular details like make, model, OS, serial number, connectivity and communication flows.
- Automate real-time medical asset inventory to free up clinical engineering resources for higher-value tasks
- Alert when a new medical device is discovered on the network
- identify medical devices that have been offline for extended periods of time
- Aid HTM teams in locating medical devices for maintenance or patching
- Identify clinical and security risks, such as medical devices with outdated operating systems or FDA recalls
- Integrate with CMMS and CMDB for real-time collaboration across teams
LEARN MORE